A comprehensive strategy to monitor quality consistency of Weibizhi tablets based on integrated MIR and UV spectroscopic fingerprints, a systematically quantified fingerprint method, antioxidant activities and UPLC-Q-TOF-MS chemical profiling†
Abstract
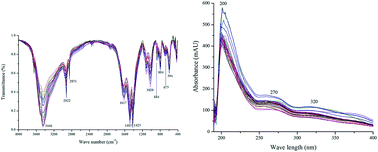
As complicated mixture systems, traditional Chinese medicines (TCM)/herbal medicines (HM) are very difficult to comprehensively investigate with regard to their quality consistency by chromatographic fingerprinting using a single detection technique. Therefore, finding a rapid, effective and comprehensive quality control method is of great importance for guaranteeing TCM/HM safety and efficacy in clinical applications. In this research, a novel combination strategy of mid-infrared (MIR) and ultra violet (UV) spectroscopic fingerprinting using Fourier transform mid-infrared (FTMIR) spectrometry and flow injection analysis (FIA) was developed and applied to monitor the quality consistency of HM in popular patent drug Weibizhi tablets (WBZT). In order to completely identify saturated and unsaturated chemical bonds for HM components in WBZT, an integrated assessment method based on MIR and UV spectroscopic fingerprinting was set up. The quality grades of 27 batches of WBZT samples from the same manufacturer were successfully discriminated by means of a systematically quantified fingerprint method (SQFM), in which both qualitative and quantitative evaluation parameters were scientifically improved on the basis of ‘Similarity’ conventionally used and ‘simple quantified ratio fingerprint method’ previously proposed. In addition, the HM chemical profiling of WBZT samples was obtained by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) in positive ion mode, providing an important chemical structure foundation for further bioactivity and quality control studies. The relationship between high performance liquid chromatography (HPLC) fingerprints and antioxidant activities of WBZT samples was established using the partial least squares regression (PLSR) method, which offers a robust predictive ability of the antioxidant activities of WBZT samples. This study demonstrates that integrated MIR and UV spectroscopic fingerprints combined with antioxidant activities can monitor TCM/HM quality consistency rapidly, effectively and comprehensively.

 Please wait while we load your content...
Please wait while we load your content...