Synthesis and antibacterial activity of ricinoleic acid glycosides†
Abstract
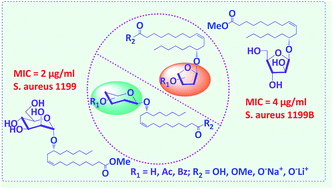
The antibacterial properties of twenty-eight novel ricinoleic acid glycosides synthesized by Koenigs–Knorr glycosylation are reported. Seven of them were found to show promising wide spectrum antibacterial activity against Gram positive bacteria of which two compounds, the mannopyranosyl- and the arabinofuranosyl derivatives, were proven effective against various non-clinical/clinical/NorA-overexpressed/resistant strains of Staphylococcus aureus as well as other Gram +ve bacteria such as Bacillus subtilis ATCC 6051 and Micrococcus luteus MTCC 2470. It was found that both the presence of the sugar and its structure are necessary and important for the compounds to be bioactive. The methyl ester protection of the carboxylic acid moiety of the ricinoleic acid unit was also found to be important for imparting good bioactivity to the molecule. Based on the membrane permeability and cell disintegration studies, these compounds are found to increase the bacterial cell membrane permeability, subsequently causing cell death.

 Please wait while we load your content...
Please wait while we load your content...