Recent progress in rhodium-catalyzed hydroaminomethylation†
Abstract
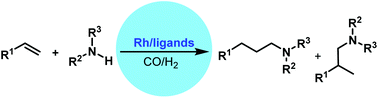
Hydroaminomethylation is a perfect reaction for converting alkenes into valuable amines with high atom economy in the presence of the syngas and amines. Significant progress has been made in the past decades; however, there still remain challenges for the control of chemo- and regioselectivity concurrently. Rhodium has proved to be a better metal in hydroaminomethylation for higher activity in hydroformylation and hydrogenation steps. Although promising results were shown by unmodified rhodium catalysts, phosphine ligand modified rhodium complexes generally displayed better activity and regioselectivity. Among the phosphorus ligands developed, tetraphosphorus ligands exhibited much better regioselectivity due to their stronger chelating ability. Apart from the phosphorus ligands, carbene and nitrogen-containing ligands have also been developed which showed good activity due to the promotion of the hydrogenation step. Although non-enantioselective hydroaminomethylation reactions have been intensively studied, reports on asymmetric hydroaminomethylation are rare. Direct asymmetric hydroaminomethylation is very challenging and only reaction systems with two different catalysts showed promising results.
- This article is part of the themed collections: Celebrating the 75th Birthday of Professor Barry Trost and 2016 Organic Chemistry Frontiers Review-type Articles

 Please wait while we load your content...
Please wait while we load your content...