Direct and metal-free oxidative amination of sp3 C–H bonds for the construction of 2-hetarylquinazolin-4(3H)-ones†
Abstract
A new method for the synthesis of 2-hetarylquinazolin-4(3H)-ones from 2-aminobenzamides and (2-azaaryl)methanes under transition metal-free conditions is described. This protocol features a wide substrate scope with a broad range of functional group tolerance under mild conditions. The oxidation of (2-azaaryl)methanes to (2-azaaryl)methanals is proposed as the key step in this transformation.
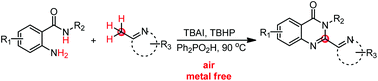

 Please wait while we load your content...
Please wait while we load your content...