Pd-catalyzed aminocarbonylation of alkynes with amines using Co2(CO)8 as a carbonyl source†
Abstract
A simple and mild protocol for the synthesis of 2-ynamides by palladium-catalyzed aminocarbonylation of alkynes was developed. The reaction proceeded at room temperature in air, utilizing Co2(CO)8 as a carbonyl source. This aminocarbonylation reaction features mild reaction conditions and good tolerance of substrates especially including aryl amines and primary amines.
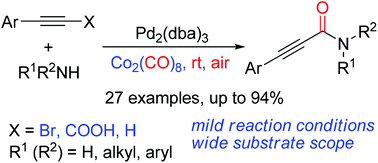

 Please wait while we load your content...
Please wait while we load your content...