The photochemical thiol–ene reaction as a versatile method for the synthesis of glutathione S-conjugates targeting the bacterial potassium efflux system Kef†
Abstract
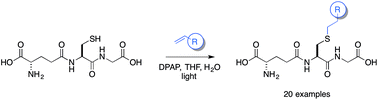
The thiol–ene coupling reaction is emerging as an important conjugation reaction that is suitable for use in a biological setting. Here, we explore the utility of this reaction for the synthesis of glutathione-S-conjugates (GSX) and present a general, operationally simple, protocol with a wide substrate scope. The GSX afforded are an important class of compounds and provide invaluable molecular tools to study glutathione-binding proteins. In this study we apply the diverse library of GSX synthesised to further our understanding of the structural requirements for binding to the glutathione-binding protein, Kef, a bacterial K+ efflux system, found in many bacterial pathogens. This system is vital to the survival of bacteria upon exposure to electrophiles, and plays an essential role in the maintenance of intracellular pH and K+ homeostasis. Consequently, Kef is an appealing target for the development of novel antibacterial drugs.


 Please wait while we load your content...
Please wait while we load your content...