Chemoselective catalytic reduction of conjugated α,β-unsaturated ketones to saturated ketones via a hydroboration/protodeboronation strategy†
Abstract
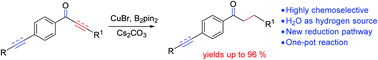
A novel copper-catalyzed chemoselective reduction of a carbon–carbon double or triple bond to a carbon–carbon single bond in α,β-unsaturated ketones is developed. This reaction proceeds under hydrogen gas or stoichiometric metal hydride free conditions. Saturated ketones were obtained in good to excellent yields with a broad substrate scope. Mechanistic studies reveal that the two hydrogen atoms come from H2O in the system. Thus this reaction represents a highly efficient, and remarkably chemoselective strategy.

 Please wait while we load your content...
Please wait while we load your content...