New developments of ketonitrones in organic synthesis†
Abstract
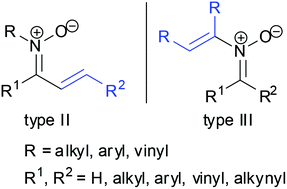
Nitrone chemistry has been widely developed for a long time and many reviews have outlined its rich transformation chemistry. However, most of these reviews involve aldonitrones due to their easy preparation and high reactivity. In recent years, N-substituents α,β-unsaturated ketonitrones and N-vinyl nitrones have attracted much attention of synthetic chemists to develop novel methodologies for their preparation and transformation. Various properties and new transformations of these nitrones have been discovered and applied into the synthesis of important heterocycle scaffolds. This review describes some recent breakthroughs in the development of new transformation of these nitrones. These new reactions include novel strategies for preparation of N-substituents α,β-unsaturated ketonitrones or N-vinyl nitrones, new rearrangement of nitrones, and reaction of nitrones with dipolarophiles such as alkynes, allenes and isocyanates.

 Please wait while we load your content...
Please wait while we load your content...