Poly(amidoamine)–BSA conjugates synthesised by Michael addition reaction retained enzymatic activity†
Abstract
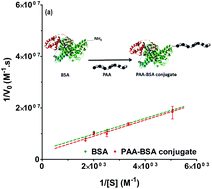
Polymer–protein conjugates are key to overcome some of the therapeutic protein limitations, including inefficient intracellular delivery. Poly(amidoamine)s are bioresponsive polyelectrolytes, which can form complexes with proteins and promote their delivery into the cytosol of cells. To investigate if conjugation would affect the activity of the protein, two poly(amidoamine)–BSA conjugates were synthesised using a “grafted to” method and Michael addition reaction. Following purification, the conjugates were characterised by electrophoresis, size exclusion chromatography (Mn(C1) = 140.7 kDa; Mn(C2) = 218.6 kDa) and light scattering (Dh(C1) = 37.5 nm; Dh(C2) = 75.1 nm). As a result of the conjugation with the cationic polymer, the conjugates had a positive zeta potential (ζ(C1) = +15.4 mV; ζ(C2) = +20.2 mV). TNBS assays demonstrated that 16% to 25% of the protein amine groups were modified and HPLC analysis indicated that the amount of protein in the conjugate was 0.76 mg of BSA mg−1 of PAA (C1) and 0.43 mg of BSA mg−1 of PAA (C2). Enzymatic assays indicated the conjugates displayed an esterase activity similar (C1) or reduced ∼35% (C2) compared to BSA. Altogether the results demonstrated that the conjugation of poly(amidoamine)s to a model protein can lead to the formation of bioconjugates that retain the enzymatic activity of the native protein. Such conjugates could have some application in protein delivery and enzyme engineering for biocatalysis and biosensors.


 Please wait while we load your content...
Please wait while we load your content...