Biodegradable poly(amidoamine)s with uniform degradation fragments via sequence-controlled macromonomers†
Abstract
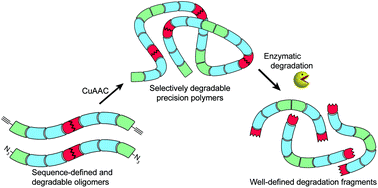
A new and general strategy for the synthesis of high molecular weight, sequence-controlled and selectively degradable poly(amidoamine)s is presented that employs solid-phase synthesis for incorporating degradable linkers at predefined positions within macromonomers. Subsequent molecular weight expansion via Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC)-mediated addition polymerization yields polymers up to an average Mn of 21 kDa. Control of the number and position of degradable linkers within the polymer backbone thus translates into complete and highly selective enzymatic fragmentation down to uniform degradation products. Hence, the control and selectivity of fragmentation now accessible with our strategy can further promote the development of degradable polymers within diagnostic and therapeutic applications.


 Please wait while we load your content...
Please wait while we load your content...