The effects of Lewis acid complexation on type I radical photoinitiators and implications for pulsed laser polymerization†
Abstract
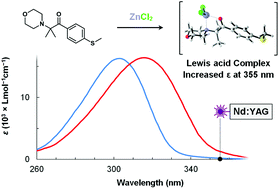
In the present work, we examine the effects of zinc chloride (ZnCl2) and aluminium chloride (AlCl3) complexation on the photochemistry of two well-known type I photoinitiators, methyl-4′-(methylthio)-2-morpholinopropiophenone (MMMP) and 2,2-dimethoxy-2-phenylacetophenone (DMPA). High-level ab initio calculations and experimental results demonstrate that Lewis acid complexation has a significant effect on the individual processes that comprise radical photoinitiation. Theoretical calculations predict that ZnCl2 coordinates to MMMP and DMPA to form thermodynamically stable bidentate ketone–amine and ketone–ether chelates, respectively. Meanwhile, the AlCl2+ cation coordinates to MMMP and DMPA to form a tridentate ether–amine–ketone chelate and a bidentate ketone–ether chelate, respectively. We found that addition of ZnCl2 and AlCl3 to solutions containing MMMP significantly increase its molar extinction coefficient (ε) between 350–360 nm. In contrast, the complexation of either ZnCl2 or AlCl3 to DMPA slightly reduces the value of ε in the 350–360 nm range. Time dependent density functional theory (TD-DFT) calculations demonstrate that Lewis acid complexation blue shifts the nπ* excitations of both DMPA and MMMP, while concurrently red shifting the ππ* transitions. Complexation also significantly alters the stability and reactivity of the photoinitiator fragment radicals. Lewis acid complexation localizes and destabilizes acyl radicals, resulting in significantly increased reactivity towards methyl methacrylate (MMA). In contrast, complexation of Lewis acids dramatically reduces the reactivity of the morpholine substituted isopropyl radical and the dimethoxyphenyl radical towards MMA. Alternative complexation at the methyl ester side-chain of MMA has a beneficial effect on the reactivity of all fragments, increasing addition rate coefficients by 2–4 orders of magnitude. We discuss some of the important implications of these findings for pulsed laser polymerization (PLP), and acetophenone photochemistry more generally.


 Please wait while we load your content...
Please wait while we load your content...