Impact of a minority enantiomer on the polymerization of alanine-based isocyanides with an oligothiophene pendant†
Abstract
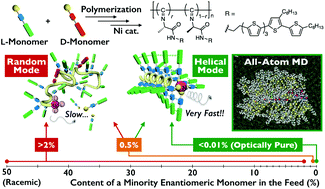
L- and D-Alanine-based enantiomeric isocyanides bearing a quinquethiophene pendant group were (co)polymerized using a nickel catalyst. The influences of the monomer feed ratio on the polymerization kinetics and the resulting polymer structures, including molecular weights and backbone conformations, were investigated by chromatography, circular dichroism spectroscopy, atomic force microscopy (AFM) and all-atom molecular dynamics simulations. For polymerization of the enantiopure monomer, the chain growth reaction was almost complete within a few minutes and yielded a one-handed helical polyisocyanide with a molecular weight of more than 1 × 107 g mol−1. The polymer single chains of micrometer-order length were directly observed by high-resolution AFM. When the polymerization feed contained 9 mol% of the antipode comonomer, the monomer consumption rate and polymer molecular weight decreased to ca. one seven-hundredth and one one-hundredth of the values obtained in the enantiopure system, respectively. We also found that the polymer containing only 2 mol% of antipode units did not adopt a helical structure at all and possessed a totally random-coil conformation. The experimental and simulation study revealed that cooperative intramolecular interactions over a long-range homochiral sequence of more than 50 repeating units were necessary for maintaining a helical conformation. These interactions included hydrogen-bonding between amide groups, and π–π stacking between quinquethiophene pendants. This cooperativity provided a favorable interaction between an inserted monomer and a growing chain end, which would likely play an important role to promote the monomer coordination to the nickel center and to accelerate the polymerization reaction.

 Please wait while we load your content...
Please wait while we load your content...