Redox-triggered crosslinking of a degradable polymer†
Abstract
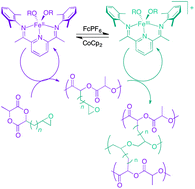
A unique redox-triggered crosslinking reaction is disclosed that capitalizes on the orthogonal reactivity of an iron-based catalyst for the ring opening polymerization of cyclic diesters and epoxides. Synthesis of an epoxide-functionalized cyclic diester is described, which undergoes chemoselective ring opening of the cyclic diester functional group when the iron catalyst is in the iron(II) oxidation state. Upon one electron oxidation of the catalyst, epoxide polymerization is triggered, which results in chemical crosslinking of the polymer. Cross-linked polymer is also obtained, albeit with different crosslinking densities, when epoxide-functionalized cyclic diester is first exposed to an iron(III) catalyst to initiate epoxide polymerization followed by one electron reduction of the catalyst to trigger crosslinking reactions that result from cyclic diester polymerization. Copolymerization reactions with lactide were carried out with the catalyst in the iron(II) oxidation state, and catalyst oxidation led to cross-linked polymers that demonstrated significantly different thermal properties compared to poly(lactic acid).

 Please wait while we load your content...
Please wait while we load your content...