Synergetic enhancement of antitumor efficacy with charge-reversal and reduction-sensitive polymer micelles†
Abstract
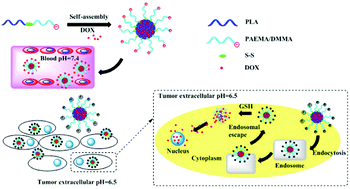
The important characteristics of a nanoparticle-based anticancer drug delivery system at least include high stability in blood, highly efficient tumor cellular uptake and stimuli-responsive release of the anticancer drug inside the tumor cells. We here report a nanomicellar drug delivery system via self-assembly of PLA-SS-PAEMA/DMMA, an amphiphilic block copolymer composed of a poly(lactide) block and a poly(PAEMA/DMMA) block with disulfide linkage. Doxorubicin (DOX) was loaded into the micelles with a high drug-loading efficiency (10.6%, LE) and entrapment efficiency (59.5%, EE). In a neutral environment, PLA-SS-PAEMA/DMMA micelles are negatively charged, which ensures low adsorption of protein in the blood and excellent hemocompatibility. The surface charges of the micelles convert to positive under slightly acidic conditions at the tumor site, which improves the uptake of the micelles by the tumor cells. Once the drug-loaded micelles enter into the tumor cells, rapid release of DOX is triggered by reductive agents inside the tumor cells, such as GSH. In comparison to single responsive DOX-loaded PLA-CC-PAEMA/DMMA micelles and DOX-loaded PLA-SS-PAEMA/SA micelles, DOX-loaded PLA-SS-PAEMA/DMMA micelles showed higher cytotoxicity against HeLa cells due to the combinational effect of charge-reversal on cellular uptake and reduction-sensitivity on intracellular DOX release. The synergetic enhancement of antitumor efficacy by charge-reversal and reduction-sensitivity in DOX-loaded PLA-SS-PAEMA/DMMA micelles was further revealed by CLSM and flow cytometry analysis.

 Please wait while we load your content...
Please wait while we load your content...