Efficient synthesis of 2-methylene-4-phenyl-1,3-dioxolane, a cyclic ketene acetal for controlling the NMP of methyl methacrylate and conferring tunable degradability†
Abstract
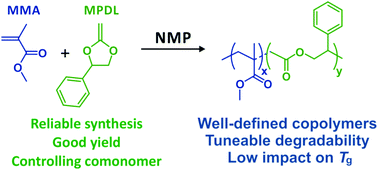
The efficient and reliable synthesis of 2-methylene-4-phenyl-1,3-dioxolane (MPDL), a highly effective cyclic ketene acetal for radical ring-opening polymerization, was reported from three different acetal halides and thoroughly characterized by 1H and 13C NMR spectroscopy, IR spectroscopy, elemental analysis and mass spectrometry. MPDL was then employed as a controlling comonomer for nitroxide-mediated polymerization using methyl methacrylate (MMA) as the principal monomer to produce well-defined, degradable PMMA-rich copolymers (Mn ∼ 20–30 kg mol−1, Đ = 1.3–1.4) when sufficient MPDL was initially introduced into the monomer feed (fMPDL,0 > 0.2). Hydrolytic degradation was tuned by varying the amount of MPDL in the monomer feed. The insertion of MPDL into the polymethacrylate backbone only moderately affected the glass transition temperature compared to the poly(methyl methacrylate) homopolymer, while giving low molar mass degradation products after hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...