Visible-light induced controlled radical polymerization of methacrylates with Cu(dap)2Cl as a photoredox catalyst†
Abstract
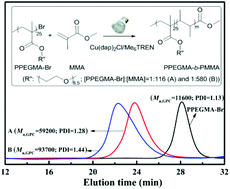
With ethyl-α-bromophenylacetate (EBPA) as an initiator and Cu(dap)2Cl (dap = 2,9-bis(p-anisyl)-1,10-phenanthroline) as a photoredox catalyst, controlled radical polymerizations of poly(ethylene glycol)methyl ether methacrylate (PEGMA) and methyl methacrylate (MMA) are demonstrated under LED light lamp irradiation (4500 μW cm−2@420 nm). The catalysis cycles proceed in the presence of N,N-dimethylaniline (DMA) or tris[2-(dimethylamino)ethyl]amine (Me6TREN), which could serve as reductants to regenerate a Cu(I) complex from the oxidized Cu(II) complex. In addition, Me6TREN plays another important role as an efficient ligand for the copper-based photopolymerization of methacrylate monomers. Good linear evolution of molecular weight (Mn) with monomer conversion is observed under the optimized conditions. Specifically, with [PEGMA] : [EBPA] : [Cu(dap)2Cl] : [Me6TREN] = 31 : 1 : 0.015 : (0.15–0.45), poly(PEGMA) (PPEGMA) with polydispersity indexes (PDI) as low as 1.15 are obtained. To further verify the living nature of this system, block copolymers of PPEGMA-b-PMMA with high molecular weights and narrow molecular weight distributions (Mn,GPC = 59 200 g mol−1, PDI = 1.28 and Mn,GPC = 93 700 g mol−1, PDI = 1.44, respectively) are prepared using PPEGMA-Br (Mn,GPC = 11 600 g mol−1; PDI = 1.13) as a macroinitiator. The polymers produced with Cu(dap)2Cl/Me6TREN as a catalyst and the PMMA obtained with Cu(dap)2Cl/DMA as a catalyst are colorless which is different from the heterogeneously catalyzed ATRP for its notorious Cu metal residue.

 Please wait while we load your content...
Please wait while we load your content...