The ring-opening polymerization of ε-caprolactone and l-lactide using aluminum complexes bearing benzothiazole ligands as catalysts†
Abstract
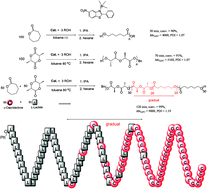
A series of aluminum complexes bearing benzothiazole ligands was synthesized and the ring-opening polymerization of ε-caprolactone (CL) and L-lactide (LA) using these aluminum complexes as catalysts was studied. The polymerization results revealed that the electron withdrawing groups increased the polymerization rate of the CL and LA polymerization. Steric bulky groups increased the polymerization rate of the CL polymerization but reduced the rate of the LA polymerization. The results also revealed that PLA-gradual-PCL that included PLA-(random-PLA-PCL)-PCL was synthesized by a one-pot synthesis, although the rate of the CL polymerization was higher than that of the LA polymerization. In addition, the results demonstrated that the catalytic activity of the Al complexes bearing benzothiazole ligands was higher than that of other Al complexes bearing heterocyclic amine ligands.

 Please wait while we load your content...
Please wait while we load your content...