Multifunctional cellulose esters by olefin cross-metathesis and thiol-Michael addition†
Abstract
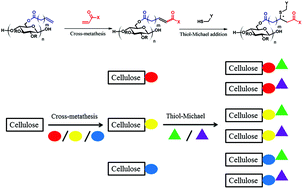
Olefin cross-metathesis (CM) has been shown to be a versatile, mild, modular, and efficient approach to polysaccharide modification. One issue with regard to this approach is the susceptibility of the initial α,β-unsaturated CM derivatives to H-atom abstraction in the γ-position, followed by radical recombination that leads to insoluble, crosslinked products. In our original approach, we resolved this problem through removing the offending unsaturation by hydrogenation. In the current study, we describe a method to exploit these reactive conjugated olefins, by post-CM thiol-Michael addition, thereby appending additional functionality. CM substrates and thiols bearing various functional groups were combined and reacted, employing amine catalysis. Up to 100% conversion was achieved under proper conditions (e.g. catalyst and reaction time), with minimal side reactions observed. The combination of the two modular reactions creates versatile access to cellulose derivatives equipped with a wide diversity of functional groups.

 Please wait while we load your content...
Please wait while we load your content...