“DNA–Teflon” sequence-controlled polymers†
Abstract
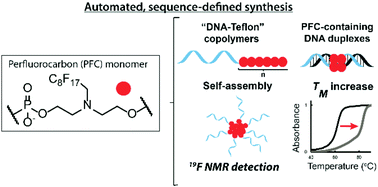
Perfluorocarbons (PFCs) are a promising class of molecules for medical applications: they are detectable through 19F nuclear magnetic resonance (NMR) and they assemble separately from water or lipophilic phases, thus bringing unique supramolecular interactions into nanostructures. We report the ready insertion of PFCs into nucleic acids, as well as non-natural polymers in a sequence-defined fashion. This is achieved via an automated and efficient synthetic pathway using phosphoramidite chemistry. Modulating the PFC tail length of “DNA–Teflon” block copolymers resulted in micelles that are almost monodisperse, have a low critical micelle concentration (CMC), are traceable by 19F NMR and are responsive to external stimuli. Strong fluorine–fluorine interactions in DNA duplexes allowed remarkable melting temperature increases and provided nuclease resistance. Finally, PFC insertion into siRNA was achieved, and the conjugates were efficient for gene silencing, outlining that these modifications are highly suitable for oligonucleotide therapeutics and bioimaging tools.

 Please wait while we load your content...
Please wait while we load your content...