Spread and set silicone–boronic acid elastomers†
Abstract
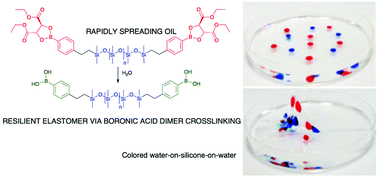
The ability of boronic acids to form complexes with a variety of ligands, including other boronic acids, has been utilized to crosslink polymers and to chromatographically separate saccharides, among other applications. It was anticipated that the formation of such complexes could be used to pin boronic acids to aqueous interfaces. Silicone–boronic acid polymers, protected as esters, were synthesized using hydrosilylation. Exposure to moisture led to deprotection of tartrate- and, at a slower rate, catechol-protected silicone boronates to give the free silicone boronic acids. Surprisingly, this deprotection was accompanied by the transformation of the silicone polymer from a liquid to a soft, elastic film (Young's modulus 150–170 kPa). Protected silicone boronates were found to be extremely efficient at rapidly spreading across water: partial hydrolysis anchored the robust, thin (<2 μm) film to the interface, which led to the formation of strong, thin silicone elastomer films. Film stability was decreased in the presence of competitive ligands in the aqueous subphase, including glycerol, phosphate, Tris, or higher pH, all of which disrupted the boronic acid: boronic acid complexation. Newly introduced water droplets on top of the film were encapsulated by the highly mobile tartrate-protected silicone boronic acid, permitting the formation of stable, stacked water droplets. The strength and behavior of self-assembled stimuli-responsive silicone materials could be tailored through a combination of boronic acid density on the silicone and the use of various analytes and conditions known to impact the coordination and ionization state of boronic acids.

 Please wait while we load your content...
Please wait while we load your content...