Optically active helical vinylbiphenyl polymers with reversible thermally induced stereomutation†
Abstract
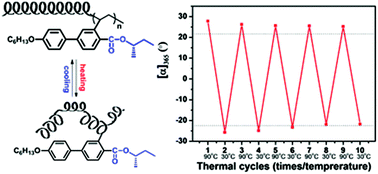
A series of novel chiral vinylbiphenyl monomers, (+)-2-[(S)-alkoxycarbonyl]-5-(4′-hexyloxyphenyl)styrene (S-(+)-I-Mm, m = 0, 1, 2, 3)/(−)-2-[(R)-sec-butyloxycarbonyl]-5-(4′-hexyloxyphenyl)styrene (R-(−)-I-M0), were designed and synthesized to search for new building blocks of optically active helical polymers. They were converted to the corresponding polymers, S-(+)-I-Pm/R-(−)-I-P0, via radical polymerization. The polymers display chiroptical properties obviously distinct from those of the monomers. The specific optical rotation of S-(+)-I-P0 scales up with molecular weight at the beginning and then levels off. The prefactor (ρ = Rg/Rh) of S-(+)-I-P0 in tetrahydrofuran at 30 °C is 2.38, indicating a stiffened polymer main chain. Changing the configuration of the stereocenter in the chiral tails as in S-(+)-I-M0/R-(−)-I-M0 leads to an inversion of optical rotation for both monomers and polymers. These results suggest that the biphenyl pendants are able to drive a polyethylene backbone to adopt an extended helical conformation, the twist sense of which is governed by the chiral tails of pendants. When the stereocenter goes far away from the polymer backbone, the optical rotation strength of polymers becomes weakened, implying the reduced asymmetric coupling between the polymer backbone and the chiral pendant groups. Unlike the previously reported helical vinylterphenyl polymers, the sign of optical rotation of S-(+)-I-Pm does not alternate with the variation of the distance between the stereocenter and the polymer backbone. It is probably attributed to the direct connection of the chiral alkoxycarbonyl group to the polymer backbone due to the absence of a rigid spacer. Such a small structural modification also endows the polymers with the dynamic nature of helical conformation, as implied by the reversible thermally induced stereomutation of polymers in aromatic solvents and the relatively lower glass transition temperature compared with their terphenyl-based counterparts.

 Please wait while we load your content...
Please wait while we load your content...