The role of local chemical hardness and van der Waals interactions in the anionic polymerization of alkyl cyanoacrylates†
Abstract
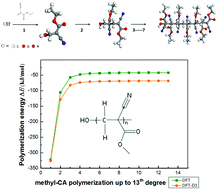
Because of their unique adhesive properties, cyanoacrylates (CA) have various applications in many technological fields. We therefore study the anionic polymerization of five types of CAs (methyl-CA, ethyl-CA, allyl-CA, 2-phenylethyl-CA and β-methoxy-CA) up to the addition of seven monomers, using density functional theory (DFT) and DFT with dispersion correction (DFT-D3). We found that the polymerization reactions are exothermic and become nearly constant after the attachment of three monomers, which means that all of these alkyl CAs undergo steady anionic polymerization until terminated by a cation. We examine the factors that can explain this energetic trend. Polymerization energies of the CAs from DFT-D3 show consistent stabilization relative to DFT, underlining the importance of intra-chain dispersion interactions. Population analysis shows that around 97% of net anionic charge is always localized between the latest added monomer's cyano and carbonyl groups, regardless of chain length, and this local chemical hardness can account for the computed energetics. The HOMO–LUMO energy gap of the CA polymers decreases as the degree of polymerization increases, with HOMO and LUMO energies becoming equal from the ninth degree of methyl-CA polymerization onwards, but this does not affect the charge distribution or consequently the polymerization reaction.

 Please wait while we load your content...
Please wait while we load your content...