How does dense phase CO2 influence the phase behaviour of block copolymers synthesised by dispersion polymerisation?†
Abstract
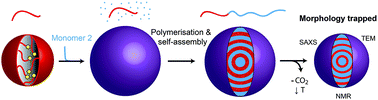
Block copolymers synthesised in supercritical CO2 dispersion undergo in situ self-assembly which can result in a range of nanostructured microparticles. However, our previous study revealed that copolymers with different block combinations possessed different microphase separated morphologies at identical block volume fractions. In this paper, we follow up those initial observations. By examining the phase behaviour of a selection of structurally diverse block copolymers, we explore the structural factors which influence the conflicting self-assembly behaviours. The composition dependence of the morphology is found to be strongly related to the CO2-philicity of the second block relative to poly(methyl methacrylate) (PMMA). Whilst PMMA-b-poly(benzyl methacrylate) (PBzMA) and PMMA-b-poly(N,N-dimethylaminoethylmethacrylate) (PDMAEMA) phase behaviour follows traditional diblock copolymer phase diagrams, PMMA-b-poly(styrene) (PS) and PMMA-b-poly(4-vinyl pyridine) (P4VP), which comprise blocks with the greatest contrast in CO2-philicity, self-assemble into unexpected morphologies at several different block volume fractions. The morphology of these copolymers in the microparticulate form was found to revert to the predicted equilibrium morphology when the microparticles were re-cast as films and thermally annealed. These findings provide strong evidence that CO2 acts as a block-selective solvent during synthesis. The CO2-selectivity was exploited to fabricate various kinetically trapped non-lamellar morphologies in symmetrical PMMA-b-PS copolymers by tuning the ratio of polymer : CO2. Our data demonstrate that CO2 can be exploited as a facile process modification to control the self-assembly of block copolymers within particles.

 Please wait while we load your content...
Please wait while we load your content...