Rapidly-cured isosorbide-based cross-linked polycarbonate elastomers†
Abstract
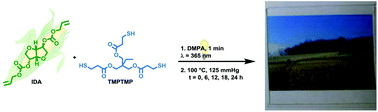
The rapid synthesis of an optically-transparent, flexible elastomer was performed utilizing the naturally-derived source, isosorbide. A novel monomer based on isosorbide (isosorbide dialloc, IDA) was prepared by installing carbonate functionalities along with external olefins for use in thiol–ene click chemistry. Cross-linked networks were created using the commercially-available cross-linker, trimethylolpropane tris(3-mercaptopropionate) (TMPTMP) and resulted in IDA-co-TMPTMP, an optically-transparent elastomer. Systematically, IDA-co-TMPTMP networks were synthesized using a photoinitiator, a UV cure time of one minute and varied post cure times (0–24 h, 125 mm Hg) at 100 °C to observe effects on mechanical, thermal and surface alterations. The mechanical properties also had limited changes with post cure time, including a modulus at 25 °C of 1.9–2.8 MPa and an elongation of 220–344%. The thermal decomposition temperatures of the networks were consistent, ca. 320 °C, while the glass transition temperature remained below room temperature for all samples. A cell viability assay and fluorescence imaging with adherent cells are also reported in this study to show the potential of the material as a biomedical substrate. A degradation study for 60 days resulted in 8.3 ± 3.5% and 97.7 ± 0.3% mass remaining under accelerated (1 M NaOH, 60 °C) and biological conditions (pH 7.4 PBS at 37 °C), respectively. This quickly-synthesized material has the potential to hydrolytically degrade into biologically-benign and environmentally-friendly by-products and may be utilized in renewable plastics and/or bioelastomer applications.


 Please wait while we load your content...
Please wait while we load your content...