Hydrolytically degradable, dendritic polyglycerol sulfate based injectable hydrogels using strain promoted azide–alkyne cycloaddition reaction†
Abstract
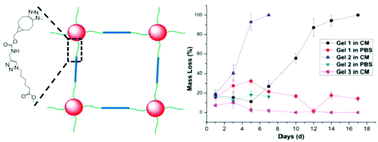
A hydrolytically degradable, cyclooctyne terminated polyethylene glycol polycaprolactone (PEG-PCL-DIC) linker has been synthesized and used to form degradable dendritic polyglycerol sulfate (dPGS)/star PEG based hydrogels. dPGS is a highly branched sulfated synthetic polymer which is analogous to heparan sulfate glycosaminoglycan (GAG). The degradation is achieved by introducing caprolactone units in the PEG. A new strained cyclooctyne–alkyne derivative is designed and has been used for the introduction of strained cyclooctynes in the linker by protecting the strained cyclooctyne using a Cu(I) catalyst and excess Cu(I) was used as a catalyst for the coupling of the remaining alkyne to the azide containing linker. Afterwards the strained cyclooctyne is regenerated by deprotecting them and used for the preparation of hydrogels. The linker is characterized by various spectroscopic methods. All the hydrogels have a highly crosslinked structure and the hydrogel formation was cytocompatible towards mouse fibroblasts L929 cells. The degradability of the hydrogels has been tested by gravimetrically monitoring the mass loss (%) in DMEM containing 10% FCS.

 Please wait while we load your content...
Please wait while we load your content...