A new luminescent lanthanide supramolecular network possessing free Lewis base sites for highly selective and sensitive Cu2+ sensing†
Abstract
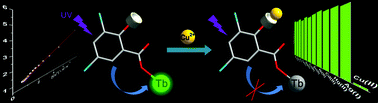
A series of new lanthanide complexes, formulated as [Ln2(DCSAL)3(H2O)11]·3DCSAL·4H2O [Ln = Eu (1), Gd (2) and Tb (3); DCSAL = 3,5-dichlorosalicylate], have been synthesized and characterized by single crystal X-ray analysis. They are dinuclear clusters and form a 3D supramolecular network via π–π stacking and halogen bonding interactions. 3 exhibits strong Tb characteristic emission, whose quantum yield is as high as 38%. Due to binding with Cu2+ ions via its Lewis acid–base interactions, 3 displayed a high selectivity and sensitivity for Cu2+ detection based on Tb3+ emission quenching. The possible quenching mechanism was further proved to be a static quenching mechanism by Stern–Volmer plots and UV-vis spectrum. More importantly, the binding constant between 3 and Cu2+ is also calculated by the Benesi–Hildebrand method, which is helpful for quantitative analysis.

 Please wait while we load your content...
Please wait while we load your content...