Role of certain amino acid residues of the coelenterazine-binding cavity in bioluminescence of light-sensitive Ca2+-regulated photoprotein berovin†
Abstract
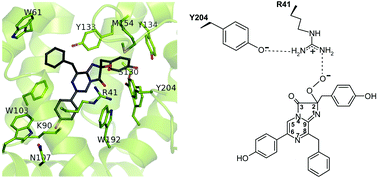
Bright bioluminescence of ctenophores is caused by Ca2+-regulated photoproteins. Although these photoproteins are functionally identical to and share many properties of cnidarian photoproteins, like aequorin and obelin, and retain the same spatial architecture, they are extremely sensitive to light, i.e. lose the ability to bioluminesce on exposure to light over the entire absorption spectrum. In addition, the degree of identity of their amino acid sequences with those of cnidarian photoproteins is only 29.4%. This suggests that the residues involved in bioluminescence of ctenophore and cnidarian photoproteins significantly differ. Here we describe the bioluminescent properties of berovin mutants with substitution of the residues located in the photoprotein internal cavity. Since the spatial structure of berovin bound with a substrate is not determined yet, to identify these residues we have modeled it with an accommodated substrate using the structures of some cnidarian Ca2+-regulated photoproteins with bound coelenterazine or coelenteramide as templates in order to obtain an adequate sampling and to take into account all possible conformers and variants for ligand–protein docking. Based on the impact of substitutions on the bioluminescent properties and model structures we speculate that within the internal cavity of ctenophore photoproteins, coelenterazine is bound as a 2-peroxy anion adduct which is stabilized owing to Coulomb interaction with a positively charged guanidinium group of Arg41 paired with Tyr204. In this case, the bioluminescence reaction is triggered by only calcium-induced conformational changes leading to the disturbance of charge–charge interaction.

 Please wait while we load your content...
Please wait while we load your content...