Solvent-induced multicolour fluorescence of amino-substituted 2,3-naphthalimides studied by fluorescence and transient absorption measurements†
Abstract
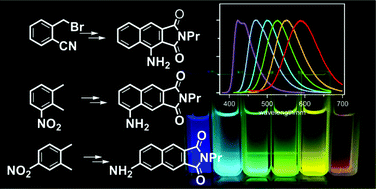
A series of amino-2,3-naphthalimide derivatives having the amino functionality at 1-, 5- and 6-positions (1ANI, 5ANI and 6ANI, respectively) were prepared, and their photophysical properties were systematically investigated based on the measurements of steady-state absorption and fluorescence spectra, fluorescence lifetimes as well as transient absorption spectra. The ANIs efficiently fluoresced in solution, and the emission spectra appreciably shifted depending on the solvent polarity. 1ANI displayed only a slight fluorescence red-shift upon increasing the solvent polarity. In contrast, 5ANI and 6ANI showed marked positive solvatofluorochromism with large Stokes shifts displaying multicolour fluorescence; the fluorescence colours of 5ANI and 6ANI varied from violet–blue in hexane to orange–red in methanol. 5ANI and 6ANI, thus, serve as micro-environment responding fluorophores. In methanol, the intensity of the fluorescence emission band of 5ANI and 6ANI significantly reduced. Based on the fluorescence quantum yields and lifetimes, and transient absorption measurements, it has been revealed that internal conversion from the S1 state of ANIs to the ground state was accelerated by the protic medium, resulting in a reduction in their fluorescence efficiency, while intersystem crossing from the S1 state to a triplet state was not responsible for the decrease of fluorescence intensity.

 Please wait while we load your content...
Please wait while we load your content...