Pd-catalyzed one-pot sequential unsymmetrical cross-coupling reactions of aryl/heteroaryl 1,2-dihalides†
Abstract
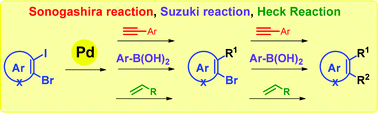
Efficient, step-economic, Pd(II)-catalyzed one-pot sequential Sonogashira/Sonogashira, Sonogashira/Suzuki, Sonogashira/Heck, Suzuki/Sonogashira, Suzuki/Suzuki, Suzuki/Heck, Heck/Sonogashira, Heck/Suzuki and Heck/Heck cross coupling reactions of sterically hindered aryl/heteroaryl 1,2-dihalides have been developed. The present methodology allows the conversion of easily available aryl/heteroaryl 1,2-dihalides into synthetically useful unsymmetrically substituted arenes/heteroarenes in good to excellent yields under mild reaction conditions. This methodology is a powerful tool for building a versatile substrate which can be utilized for the synthesis of various organic scaffolds.

 Please wait while we load your content...
Please wait while we load your content...