The evaluation of 5-amino- and 5-hydroxyuracil derivatives as potential quadruplex-forming agents†
Abstract
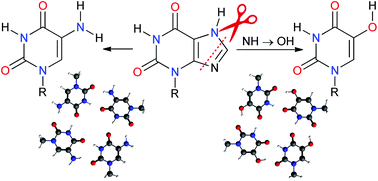
5-Substituted uracils (NH2 or OH groups in position 5) have been examined theoretically and experimentally as potential building blocks in quadruplex structures. Our high level Density Functional Theory (DFT) calculations showed that the tetramer formation and stacking energies for 5-substituted uracils are similar to the energies of purine-based xanthine (X) or guanine (G) structures. As tetrads of 5-substituted uracils cover almost exactly the same area as purine tetrads, mixed tetrads or quadruplex structures based on X or G and 5-substituted uracil motifs are possible. According to the calculations, 5-hydroxyuracil-based structures are the best candidates for experimental implementation which was corroborated by the existence of higher complexes in the mass spectra of 1-benzyl-5-hydroxyuracil. These pyrimidine-based molecules can be used as efficient building blocks in different applications including aptamers, bio-sensors or – taking into account the larger cavity in the central region of 5-hydroxyuracil structures – as an artificial ion channel.


 Please wait while we load your content...
Please wait while we load your content...