Breaking aziridines to construct morpholines with a gold(i)-catalyzed tandem ring-opening and cycloisomerization reaction†
Abstract
A convenient synthetic method for the construction of morpholine derivatives from easily available aziridines and propargyl alcohols has been successfully developed. A tandem nucleophilic ring-opening of aziridine/6-exo-dig cyclization/double bond isomerization sequence was achieved by using a single gold(I) catalyst under mild conditions. The gold(I) catalyst served as a π acid and also a σ acid to realize the dual activation of both reactants in this reaction. The obtained unsaturated morpholine products could be easily hydrogenated to achieve target morpholine derivatives with good diastereoselectivities in high yields.
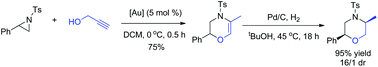


 Please wait while we load your content...
Please wait while we load your content...