Organocatalyzed asymmetric Michael addition by an efficient bifunctional carbohydrate–thiourea hybrid with mechanistic DFT analysis†
Abstract
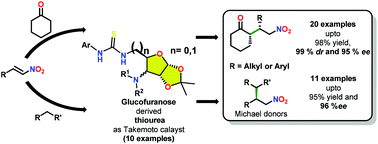
A series of thiourea based bifunctional organocatalysts having D-glucose as a core scaffold were synthesized and examined as catalysts for the asymmetric Michael addition reaction of aryl/alkyl trans-β-nitrostyrenes over cyclohexanone and other Michael donors having active methylene. Excellent enantioselectivities (<95%), diastereoselectivities (<99%), and yields (<99%) were attained under solvent free conditions using 10 mol% of 1d0. The obtained results were explained through DFT calculations using the B3LYP/6-311G(d,p)//B3LYP/6-31G(d) basic set. The QM/MM calculations revealed the role of cyclohexanone as a solvent as well as reactant in the rate determining step imparting 31.91 kcal mol−1 of energy towards the product formation.


 Please wait while we load your content...
Please wait while we load your content...