Atropisomerism in 3-arylthiazolidine-2-thiones. A combined dynamic NMR and dynamic HPLC study†
Abstract
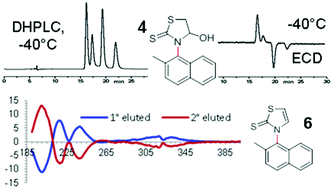
Sterically hindered 3-arylthiazolidine-2-thiones were prepared by a solvent-free reaction with arylisothiocyanates and 1,4-dithiane-2,5-diol. Atropisomerism was observed in two compounds (3 and 4, aryl = 1-naphthyl and 2-methylnaphth-1-yl), whose rotational energy barriers were measured using dynamic NMR and dynamic HPLC. The experimental analyses were supported by DFT calculations. Thermally stable atropisomers were obtained by dehydration of compounds 3 and 4 and the absolute configuration of the atropisomers of compound 6 was determined by theoretical simulation of the ECD and VCD spectra.


 Please wait while we load your content...
Please wait while we load your content...