Organocatalyzed enantioselective Michael addition/cyclization cascade reaction of 3-isothiocyanato oxindoles with arylidene malonates†
Abstract
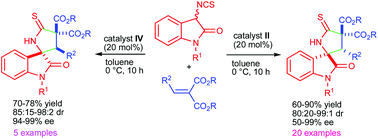
An organocatalyzed Michael addition–cyclization reaction between 3-isothiocyanatooxindoles and arylidene malonates has been developed for the synthesis of highly functionalized 3,2′-pyrrolidinyl spirooxindole derivatives. The reaction was catalyzed by a quinine derived tertiary amino-thiourea based bifunctional catalyst or its pseudo-enantiomeric quinidine derived catalyst providing both the enantiomers of the desired product. The products were obtained in high yields and with excellent diastereo- (up to 99 : 1 dr) and enantioselectivities (up to >99% ee).

 Please wait while we load your content...
Please wait while we load your content...