Divergent synthesis of indoles, oxindoles, isocoumarins and isoquinolinones by general Pd-catalyzed retro-aldol/α-arylation†
Abstract
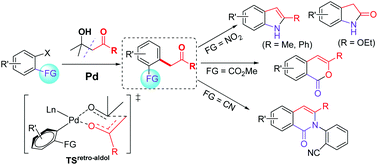
Divergent synthesis of indoles, oxindoles, isocoumarins and isoquinolinones is described in this report by using a general Pd-catalyzed tandem reaction of β-hydroxy carbonyl compounds with aryl halides bearing an ortho-nitro, -ester or -cyano substituent. A key retro-aldol/α-arylation reaction is involved that merges classic Pd cross-coupling chemistry with novel Pd-promoted retro-aldol C–C activation to produce α-arylated ketones or esters. Subsequent intramolecular condensation of the carbonyl with the ortho-synthon gives target heterocycles. The use of common, commercially available and cheap substrates and catalyst system adds additional synthetic advantages to the conceptual significance.

 Please wait while we load your content...
Please wait while we load your content...