Diastereoselective sp2–sp3 coupling of sugar enol ethers with unactivated cycloalkenes: new entries to C-branched sugars†
Abstract
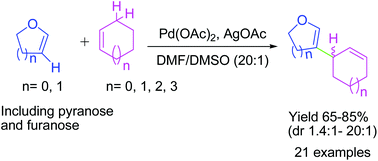
Sugar enol ethers undergo efficient coupling at C-2 with unactivated cycloalkenes under a low Pd loading affording allylic substitution products. High diastereoselectivity was observed at the allylic centre with sterically hindered substrates. Generation of a π-allyl complex by the Pd(II) catalyst via cleavage of the allylic C–H bond of the cycloalkene may be responsible for the formation of sp2–sp3 coupling products.

 Please wait while we load your content...
Please wait while we load your content...