Cross-benzoin and Stetter-type reactions mediated by KOtBu-DMF via an electron-transfer process†
Abstract
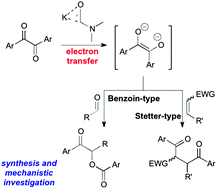
The condensation of aromatic α-diketones (benzils) with aromatic aldehydes (benzoin-type reaction) and chalcones (Stetter-type reaction) in DMF in the presence of catalytic (25 mol%) KOtBu is reported. Both types of umpolung processes proceed with good efficiency and complete chemoselectivity. On the basis of spectroscopic evidence (MS analysis) of plausible intermediates and literature reports, the occurrence of different ionic pathways have been evaluated to elucidate the mechanism of a model cross-benzoin-like reaction along with a radical route initiated by an electron-transfer process to benzil from the carbamoyl anion derived from DMF. This mechanistic investigation has culminated in a different proposal, supported by calculations and a trapping experiment, based on double electron-transfer to benzil with formation of the corresponding enediolate anion as the key reactive intermediate. A mechanistic comparison between the activation modes of benzils in KOtBu-DMF and KOtBu-DMSO systems is also described.


 Please wait while we load your content...
Please wait while we load your content...