Three-step synthesis of l-ido-1-deoxynojirimycin derivatives by reductive amination in water, “borrowing hydrogen” under neat conditions and deprotection†
Abstract
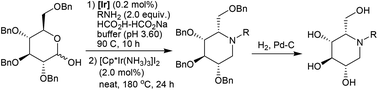
In this communication, we describe a three-step synthesis of L-ido-1-deoxynojirimycin derivatives starting from readily available 2,3,4,6-tetra-O-benzyl-D-glucopyranose via Ir-catalyzed reductive amination in water, “borrowing hydrogen” under neat conditions, and Pd-catalyzed debenzylation.

 Please wait while we load your content...
Please wait while we load your content...