Access to site-specific Fc–cRGD peptide conjugates through streamlined expressed protein ligation†
Abstract
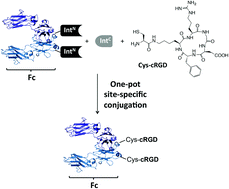
An ideal drug should be highly effective, non-toxic and be delivered by a convenient and painless single dose. We are still far from such optimal treatment but peptides, with their high target selectivity and low toxicity profiles, provide a very attractive platform from which to strive towards it. One of the major limitations of peptide drugs is their high clearance rates, which limit dosage regimen options. Conjugation to antibody Fc domains is a viable strategy to improve peptide stability by increasing their hydrodynamic radius and hijacking the Fc recycling pathway. We report the use of a split-intein based semi-synthetic approach to site-specifically conjugate a synthetic integrin binding peptide to an Fc domain. The strategy described here allows conjugating synthetic peptides to Fc domains, which is not possible via genetic methods, fully maintaining the ability of both the Fc domain and the bioactive peptide to interact with their binding partners.


 Please wait while we load your content...
Please wait while we load your content...