Characterisation of 6-DMATSMo from Micromonospora olivasterospora leading to identification of the divergence in enantioselectivity, regioselectivity and multiple prenylation of tryptophan prenyltransferases†
Abstract
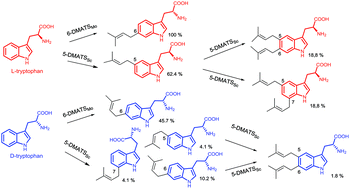
Prenylated secondary metabolites including indole derivatives usually demonstrate improved biological and pharmacological activities, which make them promising candidates for drug discovery and development. The transfer reactions of a prenyl moiety from a prenyl donor, e.g. dimethylallyl diphosphate (DMAPP), to an acceptor is catalysed by prenyltransferases. One special group of such enzymes uses DMAPP and tryptophan as substrates with dimethylallyltryptophans as reaction products and functions therefore as dimethylallyltryptophan synthases (DMATSs). Sequence homology search with known tryptophan prenyltransferases from Streptomyces led to identification of a putative prenyltransferase gene MolI14.36 in Micromonospora olivasterospora. Expression and biochemical investigations revealed that MolI14.36 acts as a tryptophan C6-prenyltransferase (6-DMATSMo). Study on substrate specificity of 6-DMATSMo displayed a significantly high activity towards D-tryptophan, which prompted us to carry out comparative studies on enantioselectivity, regioselectivity and multiple prenylation ability of additional DMATSs including FgaPT2, 5-DMATS, 5-DMATSSc, 6-DMATSSv, 6-DMATSSa and 7-DMATS towards L- and D-isomers of tryptophan and their analogues. The relative activities of the tested enzymes towards D-tryptophan differ clearly from each other. Incubation of L-, D-isomers or the racemates of 5-, 6- and 7-methyltryptophan revealed distinctly different preferences of the DMATS enzymes. Interestingly, 6-DMATSMo and 5-DMATSSc accepted 5-methyl-D-tryptophan much better than the L-enantiomer. Furthermore, the conversion yields of the D-isomers were strongly inhibited in the reactions with racemates. More interestingly, the regioselectivities of FgaPT2, 5-DMATSSc and 7-DMATS towards D-tryptophan and its C5-methylated derivative differed clearly from those of the L-forms. In addition, both mono- and diprenylated products were clearly detected for 5-DMATSSc with L- and D-enantiomers of tryptophan and their methylated derivatives.

 Please wait while we load your content...
Please wait while we load your content...