A protecting group-free synthesis of (−)-hortonones A–C from the Inhoffen–Lythgoe diol†
Abstract
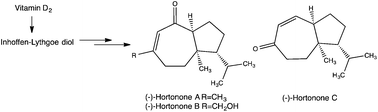
A synthesis of hortonones A–C has been accomplished from vitamin D2via the Inhoffen–Lythgoe diol without the use of protective groups. Key steps in the syntheses include a TMS-diazomethane mediated regioselective homologation of the cyclohexanone ring to a cycloheptanone moiety and a sodium naphthalenide-mediated allylic alcohol transposition. It has been found that the absolute configuration of the natural hortonones is opposite that of the synthetic material prepared from vitamin D2.

 Please wait while we load your content...
Please wait while we load your content...