A diversity-oriented approach to indolocarbazoles via Fischer indolization and olefin metathesis: total synthesis of tjipanazole D and I†
Abstract
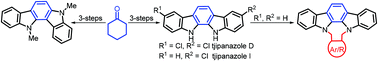
New synthetic strategies to indolocarbazoles have been reported via two-fold Fischer indolization under green conditions using L-(+)-tartaric acid and N,N-dimethyl urea. Starting with cyclohexanone, a bench-top starting material, this methodology has been extended to the total synthesis of natural products such as tjipanazoles D and I as well as the core structure of asteropusazole and racemosin B. Here, atom economical reactions like ring-closing metathesis, enyne-metathesis, and the Diels–Alder reaction have been used as key steps. Diverse strategies demonstrated here are useful in medicinal chemistry and materials science to design a library of decorated indoles.

 Please wait while we load your content...
Please wait while we load your content...