Temperature-controlled redox-neutral ruthenium(ii)-catalyzed regioselective allylation of benzamides with allylic acetates†
Abstract
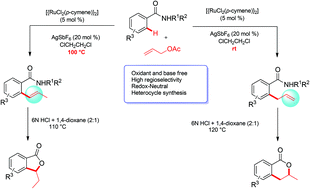
Substituted aromatic amides reacted efficiently with allylic acetates in the presence of a cationic ruthenium complex in ClCH2CH2Cl at room temperature providing ortho allylated benzamides in a highly regioselective manner without any oxidant and base. The whole catalytic reaction occurred in a Ru(II) oxidation state and thus the oxidation step is avoided. By tuning the reaction temperature, ortho allyl and vinyl benzamides were prepared exclusively. Later, ortho allyl and vinylated benzamides were converted into biologically useful six- and five-membered benzolactones in the presence of HCl.

 Please wait while we load your content...
Please wait while we load your content...