Design, synthesis, and biological evaluation of CXCR4 ligands†
Abstract
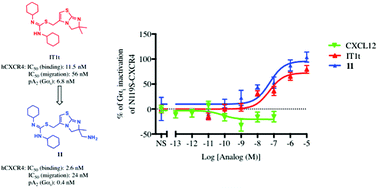
A combination of the CXCR4 inverse agonist T140 with N-terminal CXCL12 oligopeptides has produced the first nanomolar synthetic CXCR4 agonists. In these agonists, the inverse agonistic portion provides affinity whereas the N-terminal CXCL12 sequence induces receptor activation. Several CXCR4 crystal structures exist with either CVX15, an inverse agonist closely related to T140 and IT1t, a small molecule; we therefore attempted to produce another CXCL12 oligopeptide combination with IT1t. For this purpose, a primary amino group was introduced by total synthesis into one of the methyl groups of IT1t, serving as an anchoring point for the oligopeptide graft. The introduction of the oligopeptides on this analog however yielded antagonists, one compound displaying high affinity. On the other hand, the amino-substituted analogue itself proved to be an inverse agonist with a binding affinity of 2.6 nM compared to 11.5 nM for IT1t. This IT1t-like analog is hitherto one of the most potent non-peptidic CXCR4 inverse agonists reported.


 Please wait while we load your content...
Please wait while we load your content...