Biomimetic deiodination of thyroid hormones and iodothyronamines – a structure–activity relationship study†
Abstract
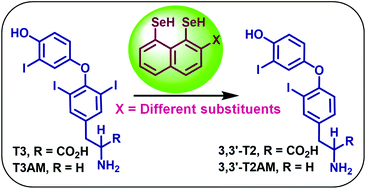
Mammalian selenoenzymes, iodothyronine deiodinases (DIOs), catalyze the tyrosyl and phenolic ring deiodination of thyroid hormones (THs) and play an important role in maintaining the TH concentration throughout the body. These enzymes also accept the decarboxylated thyroid hormone metabolites, iodothyronamines (TAMs), as substrates for deiodination. Naphthalene-based selenium and/or sulphur-containing small molecules have been shown to mediate the regioselective tyrosyl ring deiodination of thyroid hormones and their metabolites. Herein, we report on the structure–activity relationship studies of a series of peri-substituted selenium-containing naphthalene derivatives for the deiodination of thyroid hormones and iodothyronamines. Single crystal X-ray crystallographic and 77Se NMR spectroscopic studies indicated that the intramolecular Se⋯X (X = N, O and S) interactions play an important role in the deiodinase activity of the synthetic mimics. Furthermore, the decarboxylated metabolites, TAMs, have been observed to undergo slower tyrosyl ring deiodination than THs by naphthyl-based selenium and/or sulphur-containing synthetic deiodinase mimics and this has been explained on the basis of the strength of Se⋯I halogen bonding formed by THs and TAMs.

 Please wait while we load your content...
Please wait while we load your content...