5-Position-selective C–H trifluoromethylation of 8-aminoquinoline derivatives†
Abstract
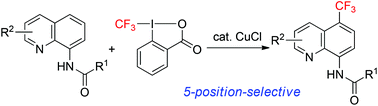
We developed a copper-catalyzed 5-position-selective C–H trifluoromethylation of 8-aminoquinoline derivatives. The reaction proceeded with high functional group tolerance under mild conditions. In the case of quinolines with an amide, carbamate, urea, or sulfonamide group at the 8-position of quinoline moieties, a radical scavenger experiment indicated that the reaction proceeded via a radical pathway. The protecting group of an 8-amidoquinoline derivative could be removed by hydrolysis. On the other hand, the trifluoromethylation of 8-aminoquinolines was also promoted by other Lewis acids as well as a copper catalyst and proceeded even in the presence of a radical scavenger. These results indicated that the trifluoromethylation of 8-aminoquinolines proceeded via a Friedel–Crafts-type reaction. Interestingly, the copper salt works as either a catalyst for the formation of a CF3 radical or a Lewis acid to promote a Friedel–Crafts-type reaction, depending on the substrate.

 Please wait while we load your content...
Please wait while we load your content...