Enantioselective synthesis of β-amino acid derivatives via nickel-promoted regioselective carboxylation of ynamides and rhodium-catalyzed asymmetric hydrogenation†
Abstract
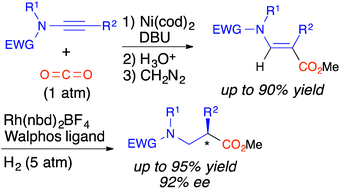
We succeeded in the development of a new method for enantioselective synthesis of α-substituted-β-amino acid derivatives. Thus, nickel(0)-promoted carboxylation of ynamide gave the α-substituted-β-aminoacrylate derivative in a highly regioselective manner. Then, rhodium-catalyzed asymmetric hydrogenation of the α-substituted β-aminoacrylate produced the corresponding α-substituted β-amino acid derivative as an optically active form.

 Please wait while we load your content...
Please wait while we load your content...