Natural product inspired design and synthesis of β-carboline and γ-lactone based molecular hybrids†
Abstract
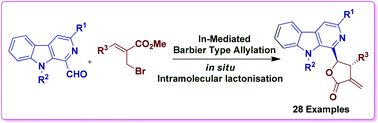
β-Carboline and γ-lactone moieties have been selected by nature as privileged scaffolds and display a wide range of pharmacological properties. Following nature, we envisaged the preparation of new β-carboline and γ-lactone based molecular hybrids incorporating both the pharmacophores. In this regard, a water-assisted In-mediated environmentally benign and easy to execute single-step tandem Barbier type allylation–lactonisation process has been devised in order to afford the targeted molecular architectures. It is anticipated that aqueous medium plays the key role in allylation as well as in the subsequent lactonisation process for the diastereo-selective synthesis of these conjugates. It is believed that water drives the reaction pathway through dual activation, it increases the electrophilic character of formyl and ester functionalities and simultaneously enhances the nucleophilic potential of the hydroxyl group to facilitate the in situ intramolecular condensation. Importantly, during this synthetic strategy no column chromatographic purification was required at any stage.

 Please wait while we load your content...
Please wait while we load your content...