A practical oxidative C–H functionalization of N-carbamoyl tetrahydro-β-carbolines with diverse potassium trifluoroborates†
Abstract
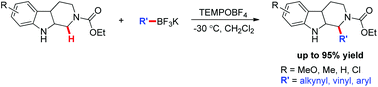
A practical and mild metal-free oxidative C–H functionalization of N-carbamoyl tetrahydro-β-carbolines has been reported. This reaction has excellent functional group tolerance, and exhibits a broad range of potassium trifluoroborate components, allowing for the facile C–H functionalization of electronically varied N-carbamoyl THCs in high efficiency with excellent regioselectivity.

 Please wait while we load your content...
Please wait while we load your content...