Horner–Wadsworth–Emmons approach to piperlongumine analogues with potent anti-cancer activity†
Abstract
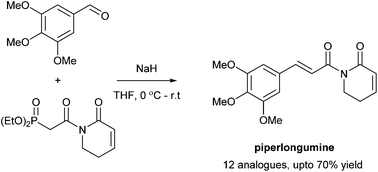
Natural products with anti-cancer activity play a vital role in lead and target discovery. We report here the synthesis and biological evaluation of the plant-derived alkaloid, piperlongumine and analogues. Using a Horner–Wadsworth–Emmons coupling approach, a selection of piperlongumine-like compounds were prepared in good overall yield from a novel phosphonoacetamide reagent. A number of the compounds displayed potent anti-cancer activity against colorectal (HCT 116) and ovarian (IGROV-1) carcinoma cell lines, via a mechanism of action which may involve ROS generation. Contrary to previous reports, no selective action in cancer cell (MRC-5) was observed for piperlongumine analogues.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...